Comparing Pharmacological and Genetic Approaches in Treating Muir-Torre Syndrome
- Jasmine Nguyen
- Jul 17
- 18 min read
Updated: Jul 21
Introduction
Muir-Torre Syndrome (MTS) is a rare inherited disorder characterized by tumors formed throughout oil glands and internal organs. These tumors can be benign and malignant and are usually found in areas such as the eyelid, scalp, and face. MTS was first discovered in 1967 by Muir and Torre but is now recognized as a variant of Lynch’s syndrome. Lynch’s syndrome increases the sucessibility of a patient towards cancer. This is because MTS is caused by germline mutations in DNA mismatch repair genes including MLH1, MSH2, MSH6, and PMS2 (Burris). Thus these mutations inhibit the body from repairing errors throughout the DNA replication process leading to genome instability and lack of integrity. Ultimately, the presence of these errors can increase the rate of tumor formation and other skin lesions. In all, MTS allows for the exploration of genetic mutations that lead to hereditary cancer and other clinical manifestations. However, new technology and drugs such as the CRISPR-Cas9 system, Isotretinoin, and Alfa-a2b can stop cancerous cells from dividing in MTS.
Primarily, MTS can be identified and detected early on through genetic testing. Specifically, a patient who may be concerned about the potential of having MTS can have their bloodwork tested for mutations within their repair genes or can have their immunohistochemistry analyzed. (Cleveland Clinic) The disease can also be seen internally, often linked with colorectal, endometrial, gastric, and urothelial cancers. Thus, there often will be poor differentiation and high levels of microsatellite instability in cells. However, most times MTS is detected through visual symptoms. Some specific skin lesions that serve as potential markers of MTS include: First, Sebaceous adenomas which are associated with eighty-five percent of MTS cases, have benign tumors that occur in sebaceous glands. These are thus places where hair is likely to grow such as the face, head, and trunk. These tumors usually look small, yellowish, or skin-colored bumps. (Cleveland Clinic) Second, is Sebaceous carcinoma which is a malignant tumor, identified as small bumps usually in the eyelids that are both dry and bleeding. (Cleveland Clinic) Next, are Fordyce spots, which are ectopic sebaceous glands that are raised in hairless areas such as the mouth. Finally, there could be Keratoacanthomas which are domes with visible blood vessels and a keratin core (Cleveland Clinic). Simply, MTS creates skin lesions that are visible over the skin and are often signs for diagnosis.
Living with MTS can be significantly hard for patients, with challenges such as constant cancer screenings and dermatology assessments. The physical effects of these skin lesions can be disfiguring to the eye, causing problems with self-esteem and social interactions in patients. Furthermore, as a hereditary disorder, they may also worry about possible inheritance to their offspring. Finally, as a variant of Lynch’s syndrome, the patient can develop cancerous tumors that lead to various significant health problems, including death. Thus, MTS can cause both psychological and physical stress for those who are diagnosed with it.
Thus, this paper explores the genetic basis, clinical examination, and management of MTS through technology and drugs. Each method will be compared and analyzed against one another, weighing both benefits and potential repercussions. Examining the mechanisms, effectiveness, and applications of each form of treatment improves the lives of MTS patients in different ways.
Thesis Statement
Muir-Torre Syndrome (MTS), a rare genetic condition characterized by mutations in DNA mismatch repair genes, poses unique treatment problems due to both internal and exterior tumor presentations. Pharmacological methods such as isotretinoin and interferon-alpha 2b can be compared against developing genetic technologies such as CRISPR-Cas9 to determine their efficacy, limits, and potential to enhance patient outcomes.
Muir-Torre Syndrome Mutation and Inheritance
As mentioned briefly before, Murri Torre Syndrome is caused by mutations in DNA mismatch repair genes including MLH1, MSH2, MSH6, and PMS2. Normal wild-type versions of
these genes are, “necessary for the maintenance of genomic stability. In a broad sense, all main functions of the MMR system, including the correction of biosynthetic errors, DNA damage surveillance, and prevention of recombination between nonidentical sequences serve this important purpose.” (Peltomäki, 2003) Thus, the loss of function of these genes can lead to malignant tumors associated with Lynch’s syndrome and MTS. Among these genes, mutations in MSH2 are the most common cause of MTS, responsible for about 90 percent of these cases up to date. Specifically, it was discovered that there was a common frameshift mutation of c.229_230delAG in MSH2’s exon two. ( Feng 2021) In other cases, deletions were found in the EpCAM gene upstream of MSH2 causing hypermethylation which reduces gene expression (Roberts 2013).
Next looking at the inheritance patterns, MTS is autosomal dominant, meaning that one copy of the gene is enough for an offspring to inherit the disease. Usually, there are several implications of being an autosomal dominant inherited syndrome. Primarily, a parent that has MTS has a fifty percent chance of passing the condition on to their offspring. Furthermore, there will be no skipping of generations. Especially since MTS has a high degree of penetrence, individuals with the genotype will develop symptoms of the syndrome, but with variable expressivity (Victor 2020),
In all, understanding both the nature of mutation and inheritance will allow for a deeper understanding of possible treatments and diagnoses for patients.
Treatment via Emerging Technology
Several emerging technologies have been present to treat MTS and other variants of Lynch syndrome. The dermatology aspect focuses on the identification and excision of sebaceous tumors, particularly those suspected of malignancy. Furthermore, hysterectomy (removal of the uterus), oophorectomy (removal of ovaries), or colectomy (bowel resection) can be conducted (Clevland Clinic). In general, there are not many therapies and treatments for hereditary non-polyposis colorectal cancer.
However, a promising method has been discovered by Shravan Kannan and Joshua J Man that implicated the use of CRISPR genome editing for genes MLH1 and MLH2. Primarily, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) as a method involves using enzymes such as CAS9 to cut DNA based on guide RNAs. The CAS9 protein specifically will lead to an area to create a double-stranded break or nicking CAS 9 to induce a single-strand break. (Zhang 2013) Researchers can then insert, repair, or edit using a template. Then, as the strand breaks are getting fixed via non-homologous end joining or homology-directed repair, the template will also be used and the code in the genome will be altered.
Thus, Kannan proposed the use of CRISPR and CAS-9 nickase to target the specific mutation clusters of MLH1 and MLH2 genes, which as mentioned prior are responsible for most MTS in patients. Specifically, the system will induce a single-stranded break in the mutated sites. Here, double-stranded breaks are intended to be minimized as they can lead to unintended insertions and deletions. Then, the cell’s homology-directed repair function will be activated with the wild-type DNA to be inserted. The repair also requires the correct wild-type DNA to be flanked by homologous arms for precise correction. Additionally, the guide RNA that is required for CAS-9 nickase would be optimized through tools such as BLAST and CASOFFFinder to target two mutated sites of Lych Syndrome variants properly: Exon 2 of MLH1 which affects the ATPase binding domain, and exon 12 of MSH2 which affects the PMS2 interaction domain. The gRNA, CAS-9 nickase, and template will all be delivered by Lentiviral vectors, which are derived from lentiviruses and modified to carry genetic material to cells. (Kannan, J Man, 2023)
Although the CRISPR Cas-9 system can directly help with MTS and Lynch Syndrome variants, there are both benefits and shortcomings to consider. Primarily, CRISPR offers a solution that is precise and efficient and is considered on as the strongest genetic editing tool (Shen 2017). It could help with all hereditary cancers and reduce the need for harsh and invasive methods that often take a toll on a patient’s health. Furthermore, it could correct the genetic defects at the root of the cause, completely stopping tumor development. However, there are also shortcomings such as off-target effects where unintended mutations could take place, leading to more harmful consequences. Additionally, there are possible long-term effects that come with the method that are not fully studied, raising questions about CRISPR’s safety and stability. Finally, there are ethical dilemmas related to the treatment that need to be addressed. Thus, while the CRISPR system shows great potential, additional research, and testing are required. Although it is an excellent strategy for addressing the underlying cause of MTS, pharmacological treatments are currently chosen due to their considerable scientific support and proven safety. However, with further research, such as Kannan's, CRISPR may become a more effective and feasible approach for treating illnesses like MTS and Lynch Syndrome.
Treatment via Oral Isotretinoin
Since the CRISPR Cas9 system is not fully researched and effective, dermatological drugs such as Isotretinoin can be used to suppress tumors and remove skin lesions in MTS. Historically, Isotretinoin was studied in the 1960s for skin cancer and later for acne vulgaris. To do this, the drug significantly reduces the amount of sebum production in the skin, prevents clogged pores, and shrinks the sebaceous glands. (Barbieri 2023) For MTS, the reduction of sebaceous glands can prevent the production of tumors and prevent the differentiation and proliferation of sebaceous cells. Although the drug does not directly treat the causes of the genetic mutation like in the CRISPR Cas9 system, it prevents the formation of external malignant tumors that can be life-threatening for patients. Isotretinoin is the primary treatment for MTS but comes with various side effects.
First, looking at the chemical structure of Isotretinoin, the drug is a derivative of Vitamin A called 13-cis retinoic acid. Because it is a variant of Vitamin A, it can go through similar processes including repairing the skin, slowing down cell turnover, and producing less clogged pores. (Gremly) The structure of Isotretinoin can be seen in Figure 1.
Here the cis configuration of the double bond seen on the chemical structure will be isomerized to trans to allow Isotretinoin to bind efficiently to receptors to eventually reduce sebaceous glands. The receptors in the glands that are targeted are called retinoic acid receptors (RAR) and the structure of Isotretinoin mimics the natural ligand of the receptors, allowing them to effectively bind. RAR receptors are ligand-controlled transcription factors that are key for multiple processes in the skin such as cell growth, differentiation, gene expression, survival, and death (le Maire). Thus, Isotretinoin is one of the only chemical structures that can bind to the RAR receptors, allowing it to be one of the most effective treatments for acne, tumors, and other skin lesions (Layton 2009). By looking at Isotretnoin’s chemical structure and its interaction with RAR, the action of mechanism and be fully understood. The specific mechanism of Isotretinoin and RAR can be seen in Figure 2 and analyzed.
The goal, of isotretinoin after the mechanism of action is to eventually cause Sebocyte apoptosis, leading to the sebum suppressive effects required for tumor prevention and other skin lesion reductions in MTS. First, the 13-cis retinoic acid is taken orally by the patient and undergoes isomerization through an Isomerase enzyme within the sebocytes and transforms into all-trans retinoic acids which bind to the RARs and Retinoid X Receptors (RXR). These receptors will form heterodimers which regulate gene expression and cause a further cascade of transcriptional activities. The activation of RAR and RXR will then influence the expression of pro-apoptotic proteins, which are key for the therapeutic effects of isotretinoin. This is done by the upregulation of Forkhead Box O3a, or FoxO3a, which is a transcription factor that drives the expression of two pro-apoptotic proteins. (Draghici 2020)
The first protein FoxO3a transcribes is the Tumor Necrosis Factor apoptosis ligand or TRAIL, which binds to receptors DR4/TRAIL-R1 and DR5/TRAIL-R2. The receptors trigger the formation of death-inducing signaling complexes (DISC) on the intracellular side of the membrane. These DICS can lead to the Caspase 8 or 3 activation. For Cascade-8 activation, the DISC will recruit procaspase-8 using a Fas-Associated Death Domain. Then, the procaspase-8 will become activated caspase-8, leading to TRAIL-mediated Sebocyte apoptosis. For Cascade 3 activation, the activation of Cascade-8 will also activate Procaspase 3, converting it into active Caspase-3. The Caspase-3 will degrade key proteins including the Poly ADP-Ribose polymerase which is involved in DNA repair and cytoskeletal proteins, leading to cell shrinkage and membrane blebbing, ultimately leading to Sebocyte Apoptosis (Pimentel 2023).
The second protein transcribed is called FOXO1, which is key for the regulation of the cell cycle and transcriptionally activating key cyclin-dependent kinase inhibitors (CKIs) including p21 and p27. These inhibitors will eventually induce the cell cycle arrest. To transcribe these elements. FOXO1 will bind to Forkhead response elements in the promoter regions of these genes. These CKIs inhibit cyclin-CDK complexes including cyclin D-CDK4/6 and cyclin E-CDK2 which play roles in the G1 phase and S phase. Thus, by halting the cyclin CDK activity, FOXO1 and the activated CKIs will pause the cell cycle progression, allowing the cell to either repair damage or enter apoptosis. (Draghici 2020) Thus, FOXO1 And TRAIL inhibit the ability of Sebocytes to proliferate and differentiate. Sebaceous activity and sebum output are reduced, ultimately lowering the amount of possible tumors and skin lesions from MTS to form. Typically for MTS, 20 milligrams of isotretinoin should be taken daily (Weiss)
Looking at the patient outcomes, a specific study conducted by UCLA Health examined low dosages of isotretinoin (20 mg) for Murri Torre Syndrome (Weiss). The case studies a 57-year-old male with MTS that presented Sebaceous adenomas and carcinomas. After the consumption of the Isotretnoin, there was a profound reduction of the skin legions and current neoplasms disappearing. Over the next two years, no lesions formed. Thus, the case displayed the role of isotretinoin in not only controlling the existing lesions but also against the development of new ones. The drug can be seen any many other cases and research as an effective maintenance therapy for MTS, providing benefits without the use of harsh and intensive treatments.
Lastly, it's important to carefully weigh the possible effects of utilizing Isotretinoin. Numerous adverse effects may result from the body having high amounts of vitamin A derivatives. Users may initially feel peeling, blisters, and dry skin, especially around the lips, nose, and mouth as there is significant cell turnover occurring in the skin. Long-term consequences may include hearing problems, such as ringing in the ears and hearing loss, as well as vision problems, such as blurriness and vision loss. Mood disorders such as sadness, mood swings, violent inclinations, and even suicidal thoughts can have other severe consequences. In addition, users may experience stomach and liver problems, as well as joint and muscle pain (Cleveland Clinic). These ultimately could affect daily physical activity and worsening of the skin temporarily patients should consider before taking Isotretinoin.
Treatment via Interferon Alfa-2a
The second drug that can be used for tumor suppression is Interferon Alfa-2a (IFN-a2b) which is a pleiotropic cytokine, able to exhibit multiple functions. Part of the I interferon family, it is a recombinant protein that is produced through DNA technology. IFN-a2b is widely used for its antiviral, antiproliferative, and immunomodulatory properties for several malignancies such as melanoma, renal cell carcinoma, and hairy cell leukemia. (Xiong 2022) For patients, it often is implemented through multiple injections and requires careful patient selection due to the potential side effects. Just like isotretinoin, the drug does not directly address the mutations of the syndrome but treats it through tumor suppression. In all, IFN-a2b has several primary and secondary effects that lead to tumor suppression and maintenance.
The first mechanism of IFN-a2b is mediated by the Janus Kinase (JAS)- signal transducers and the activators of the transcription (STAT) pathway, which are key signaling cascades in immune and cellular regulation. The JAK-STAT pathway can be seen in Figure 4. The process first starts as IFN-a2b binds to high-affinity receptors, IFNAR1 and IFNAR2 on the cell surface. This interaction then causes the receptors to dimerize and activate the JAK1 and TYK2 kinases. These will then phosphorylate specific parts inside the IFNAR1 and IFNAR2 receptors, creating docking sites for downstream signaling molecules. Once the phosphorylated receptor complex recruits STAT1 and STAT2 proteins, they are further phosphorylated by JAK1 and TYK2. The phosphorylated STAT1 and STAT2 then will form heterodimers that bind to interferon regulatory factor 9 (IRF9) and create interferon-stimulated gene factor 3 (ISGF3). Then from the cytoplasm, ISGF3 will travel into the nucleus where it binds to interferon-stimulated response elements in the promotors of genes to activate transcription. This interaction will drive expressions of hundreds of genes that cause a diverse range of downstream effects including antiviral, and antiproliferative responses, and cell death. (Xiong 2022)
The first consequence of the expression of interferon-stimulated genes (ISGs) is antiviral responses. Specifically, myxovirus resistance proteins are encoded including MX1 and MX2 which disrupt viral replication. Second, IFN-a2b upregulates genes that lead to antiproliferative responses. Specifically, proteins including p21 and p27 are made, disrupting the cyclin-dependent kinase activity and thus halting cell proliferation (this process was explained thoroughly in the Isotretnoin section). Next, IFN-a2b can cause apoptosis first through the production of proteins like Bax and TRAIL. Finally, the drug can also produce a reactive oxygen species which causes oxidative stress within tumor cells, further promoting apoptosis. (Cabellos 2022) Thus, the IFN-a2b pathway directly promotes responses against tumor growth and survival within MTS.
Along with the JACK-STAT pathway, IFN-a2b influences alternative pathways that can help with MTS. The first is the Wnt/B-catenin pathway, which normally leads to tumor cell proliferation and metastasis. Specifically, the drug inhibits the B-catenin pathway and prevents it from interacting with TCF/LEF transcription factors which disables oncogenic signaling (Ceballos 2022).
Additionally, IFN-a2b influences the Beclin1 pathway to promote autophagy, which is the process where cells break down and recycle damaged parts. Specifically in the context of tumors, the cells will degrade damaged cellular components and can cause apoptosis (Xiong 2022). Third, IFN-a2b can increase the activation of Caspase-3 and Caspase-8, which dismantle cellular structures throughout apoptosis. In all, these three alternative pathways are complementary to the JAK-STAT pathway, extending the IFN-a2b’s anti-malignant effects.
Finally, IFN-a2b can enhance immune responses through the activation of dendritic cells within tissue, macrophages, and natural killing cells. These all can modulate the tumor microenvironment by boosting the innate and adaptive immunity systems. (Xiong 2022)
Clinically, IFN-a2b is often used with isotretinoin to help manage and treat Murri Torre Syndrome. In a study in 1997, a 57-year-old man showed symptoms of MTS with a 12-year history of both external skin and internal tumors. He showed multiple yellowish and skin-colored tumors and skin lesions on his face, back of neck, and upper arms. The diagnosis of MTS was confirmed and he was prescribed both isotretinoin along INF-a2b injections three times a week. As seen in Figure 5, after three months, there was a significant reduction in skin lesions and tumors with only one cystic skin tumor developing after the isotretinoin dosage was reduced (Graefe 2000)
Despite its therapeutic promise, INF-a2b has many limitations for treating MTS. Specifically, tumor heterogeneity and mutations in the key signaling proteins including JACK, TYK2, and STAT can disrupt the pathway and reduce efficiency. For example, mutations in STAT1 and STAT2 can block the ISGF3 formation and thus, the entire JACK-STAT pathway Furthermore, there are several side effects of the drug such as flu-like symptoms, hematological toxicity, and negative neurophysictriac effects that need to be taken accounted for. In general, there are a lot of side effects of the drug that are hermetic, musculoskeletal, metabolic, hepatic, and gastronomical that are seen. The patient’s history also needs to be looked at as INF-a2b is specific for usage per patient. Thus, although the drug may be useful in treating MTS, it is more specific in selectivity for usage and has a wide range of negative effects.
Comparison of Drugs
Looking at both Isotretinoin and IFN-2ab, are both viable options for treating MTS and its various skin lesions and tumors. These two drugs are the only two generally provided for treatment. Alternative options for treatment are often harsh for the body and are more focused on physically removing the tumors and lesions. Both drugs have strengths, weaknesses, and possible improvements that can be further analyzed.
Primarily looking at Isotretinoin and the basis of its mechanism of action, the drug is effective in causing Sebcrous apoptosis which is applicable for all types of skin lesions. Furthermore, the drug is deeply researched and is both safer short and long-term. (The side effects discussed in the Isotretinoin section of the paper) The drug is applicable for most people except those with certain skin conditions including Xeroderma, dry eye syndrome, arthralgia, and eczema which can be worsened by the use of Isotretinoin. (Goulden 1994) Studies have also shown that the use of isotretinoin early on can help treat patients with MTS with few new lesions. The drug overall has a high success rate (Layton 2009). However, Isotretinoin targets only external tumors as the end effect is the reduction of sebaceous glands. The drug can thus only eliminate the skin lesions and tumors that are present in the skin and not those internally. As an effect, the drug should be prescribed to those in the early stages of Murri Torre syndrome with the initial symptoms only dealing with lesions seen on the skin. However, for those with long untreated MTS like in the second patient discussed prior, Isotretinoin only can not fully treat the symptoms. There are a few improvements for the drug as it is mostly successful in fulfilling its original purpose. However, one minor alteration that could improve Isotretinoin is its initial purging and drying of the skin and joints which can affect a patient’s daily life. To achieve this, a targeted drug delivery system using nanoparticles could maybe be used to direct Isotretinoin in affected areas which will minimize the dryness/purging in unaffected areas.
Secondarily, looking at IFN-2ab and its mechanism of action, the drug is very effective in tumor cell apoptosis both external and internal. IFN-2ab can be seen as a very efficient drug in treating MTS due to its numerous pathway interactions that lead to several immune responses including antivirus, antiproliferation, and immunomodulatory activities against tumors (Xiong 2022). Thus, it is not only limited to treating MTS but several other diseases that require a strong immune system reaction. However, although the drug is researched backed, and approved, there are several cases where IFN-2ab has worsened the case of the patients who used it. One variant of the IFN-2ab drug was discontinued because it made a 64-year-old woman’s illness significantly worse (Fenn). This may be due to IFN-2ab's ability to react in other pathways that enhance malignancy unintendedly leading to worse symptoms (Xiong 2022). There are also many sources and articles citing the various long-term side effects of the drug as well as its failure in treating the intended disease. Finally, there is high selectivity with reactions that may vary per person depending on the severity of the MTS and history (Xiong 2022) Thus, IFN-2ab has a lower success rate than Isotretinoin. However, it does accomplish the goal of removing internal malignancies that Isotretinoin lacks. Thus, further research and development of the drug can make it more effective in treating MTS. Two possible improvements can be made to make IFN-2ab greater. First, implementations can be made to limit the side effects of the drug. Because the drug is artificially created, it could be engineered alternatively to only activate specific pathways to help tumor suppression and reduce interactions with malignant pathways. Secondly, checkpoint inhibitors could be used to reduce the need for high doses that generally lead to significant side effects.
Thus, comparing both drugs, Isotretinoin and IFN-2ab can help treat those with MTS. For those early on with only skin lesions and external tumors, Isotretinoin may be more useful as there are fewer side effects and general success. However, with more severe cases with more internal tumors, IFN-2an is needed to be used as Isotretinoin is inefficient. However, IFN-2an is less useful for targeting the extra skin lesions that are not malignant due to its mechanism of action. Thus, dermatologists usually prescribe both drugs together to complement each other’s effects and limitations.
Conclusion
In all, Murri Torre Syndrome is a rare, inherited cancer syndrome that causes a variety of skin lesions, and internal, and external skin lesions to exist. Genetically, the disease is caused by mismatch repair genes including MLH1, MSH2, MSH6, and PMS2. Generally, patients diagnosed with MTS should be worried about the possible inheritance of the disease to their offspring. By understanding the genetic makeup and the systematic effect of the MTS, multiple pharmaceutical and technological treatments arise. Primarily, drugs like Isotretinoin cause sebaceous apoptosis and provide effective solutions for early-stage patients with skin lesions and external tumors. On the other hand, INF-2ab targets both internal and external tumors, offering several anti-malignant and immune system responses. However, its side effects and limited success raise many challenges. Both pharmaceutical options allow for the maintenance of the disease, eliminating the symtoms and effects, however, do not directly treat the genetic mutation that is the root of the cause.
Thus, emerging technology such as CRISPR-Cas 9 holds a promising alternative to treating the mutations causing MTS. However, there are several limitations such as limited research, ethical considerations, and the potential for off-target effects to alter its widespread clinical application. However, with time, CRISPR-Cas 9 can be a better solution than both drugs, negating their limitations and side effects and ensuring that no future symtoms arise.
In all, by comparing and analyzing these approaches, each treatment aims to treat MTS patients differently, affecting different symtoms or genetic mutations. Isotretinoin, INF-2ab, and CRISPR-Cas 9 all hold great promise in improving the patient lives of those with Murri Torre Syndrome. (Page count total: 13)
Figures
Figure 1

Figure 2
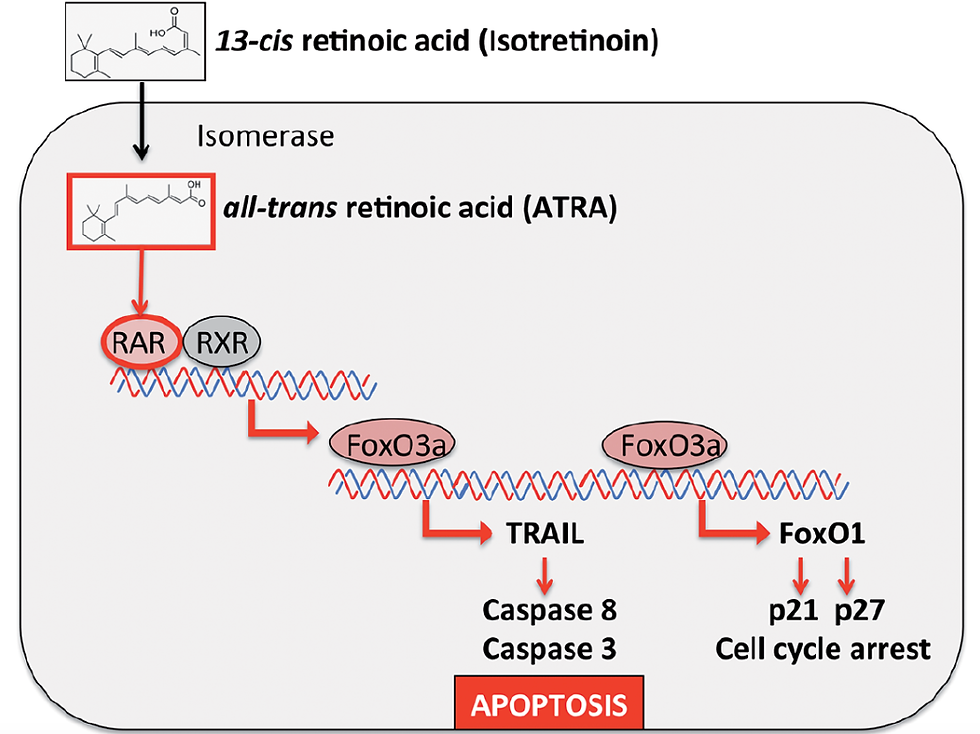
Figure 3

Figure 2 presents the case before and after the usage of Isotretinoin for Murri Torre Syndrome.
Figure 4
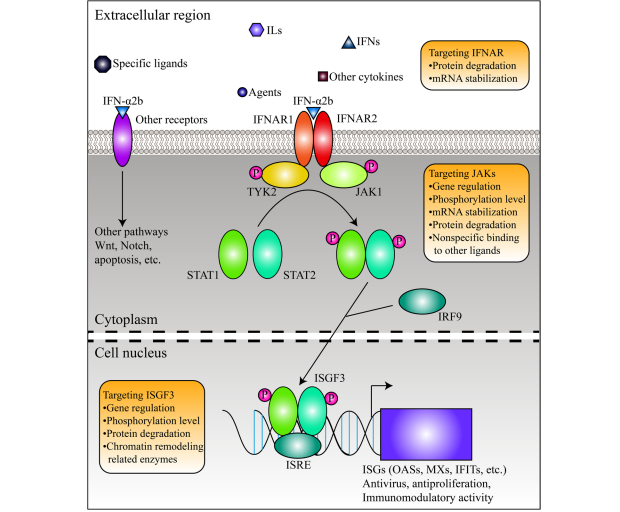
Figure 5

Bibliography
Shi, W., Yao, X., Fu, Y., and Wang, Y. “Interferon-α and Its Effects on Cancer Cell Apoptosis.” Oncology Letters, vol. 24, no. 1, May 2022, p. 235, doi:10.3892/ol.2022.13355. PMID: 35720476; PMCID: PMC9185151.
Xiong, F., Wang, Q., Wu, G. H., Liu, W. Z., Wang, B., and Chen, Y. J. “Direct and Indirect Effects of IFN-α2b in Malignancy Treatment: Not Only an Archer but Also an Arrow.” Biomarker Research, vol. 10, no. 1, Sept. 2022, p. 69, doi:10.1186/s40364-022-00415-y. PMID: 36104718; PMCID: PMC9472737.
"Interferon-Alpha-2B Treatment Overview." PubMed Central, https://pmc.ncbi.nlm.nih.gov/articles/PMC2835909/.
“Muir-Torre Syndrome.” Cleveland Clinic, https://my.clevelandclinic.org/health/diseases/25030-muir-torre-syndrome.
Feng, Y., Feng, J., and Bao, J. “Case Report: A Frameshift Mutation in MSH2 Exon 2 in a Kidney Recipient with Muir-Torre Syndrome.” Frontiers in Oncology, vol. 11, June 2021, p. 681780, doi:10.3389/fonc.2021.681780. PMID: 34249727; PMCID: PMC8264542.
Roberts, M. E., Rueda, B., Wasdahl, W. A., et al. “Lynch Syndrome-Associated Epithelial Ovarian Cancer.” Gynecologic Oncology, vol. 130, no. 2, 2013, pp. 482–487, doi:10.1016/j.ygyno.2013.06.010.
“Muir-Torre Syndrome Overview.” Medscape, https://emedicine.medscape.com/article/1093640-overview.
Graefe, T., Wollina, U., Schulz, H., and Burgdorf, W. “Muir-Torre Syndrome - Treatment with Isotretinoin and Interferon Alpha-2a Can Prevent Tumour Development.” Dermatology, vol. 200, no. 4, 2000, pp. 331–333, doi:10.1159/000018399.
“Isotretinoin Capsules.” Cleveland Clinic, https://my.clevelandclinic.org/health/drugs/19186-isotretinoin-capsules.
Le Maire, A., Alvarez, S., Shankaranarayanan, P., et al. “Retinoid Receptors and Therapeutic Applications of RAR/RXR Modulators.” Current Topics in Medicinal Chemistry, vol. 12, no. 6, 2012, pp. 505–527, doi:10.2174/156802612799436687.
“Vitamin A for Acne.” Natural Acne Clinic, https://www.naturalacneclinic.com/blog/vitamin-a-acne/#:~:text=In%20short%3A,also%20contribute%20to%20clogged%20pores.
“Isotretinoin’s Effects on Acne.” JAMA Dermatology, https://jamanetwork.com/journals/jamadermatology/fullarticle/2810450#:~:text=Isotretinoin%20works%20in%20several%20ways,Third%2C%20it%20may%20lower%20inflammation.
“Interferon Therapy and Genetic Mutations.” bioRxiv, https://www.biorxiv.org/content/10.1101/2023.06.20.545835v1.full.pdf.
Shen, S., Loh, T. J., Shen, H., Zheng, X., and Shen, H. “CRISPR as a Strong Gene Editing Tool.” BMB Reports, vol. 50, no. 1, Jan. 2017, pp. 20–24, doi:10.5483/bmbrep.2017.50.1.128. PMID: 27616359; PMCID: PMC531966.
Goulden, V., Layton, A. M., and Cunliffe, W. J. “Long-Term Safety of Isotretinoin as a Treatment for Acne Vulgaris.” The British Journal of Dermatology, vol. 131, no. 3, 1994, pp. 360–363, doi:10.1111/j.1365-2133.1994.tb08524.x.
Layton, A. “The Use of Isotretinoin in Acne.” Dermatoendocrinology, vol. 1, no. 3, May 2009, pp. 162–169, doi:10.4161/derm.1.3.9364. PMID: 20436884; PMCID: PMC2835909.

Comments